Gyntools

Gyni is a multidisciplinary AI system. It is comprised of:
Smart sampler
- for vaginal discharge sample collection and preparation.
- an automated microscope that scans the sampler’s cassette.
Cloud-based, machine learning algorithm
- trained by a unique archive of 12,000 screened & classified vaginitis conditions images.
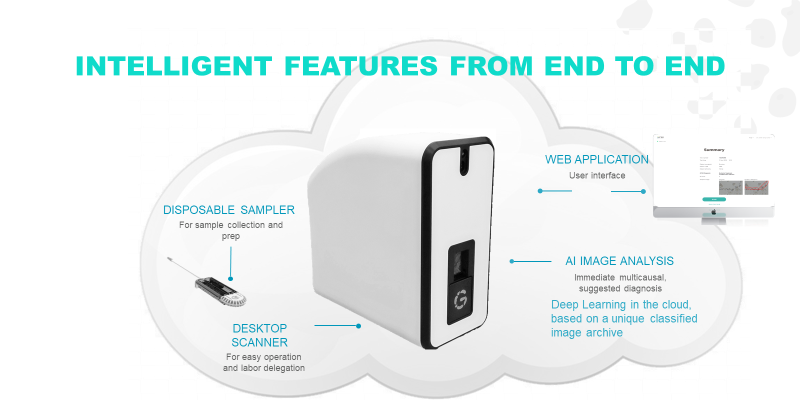
GYNI™ is the first Point-Of-Care vaginitis diagnostic tool for gynecologists and primary caregivers.
GYNI™ can diagnose the following conditions
- Bacterial Vaginosis
- Candida Albicans
- Candida Non Albicans
- Trichomonas Vaginalis
- Desquamative Inflammatory Vaginitis
- Cytolytic Vaginosis
- Vaginal Atrophy
GYNI™ Benefits
- Accurate and Rapid diagnosis of both infectious and non-infectious conditions
- One scanner can be used by multiple doctors
- Use of the scanner can be delegated to other clinic personnel
- Saving all paperwork and bureaucracy of samples sent to lab
(1) Bacterial Vaginosis (2) Candida albicans (3) Candida non-albicans (4) T. vaginalis (5) vaginal atrophy (also referred to as atrophic vaginitis or genitourinary syndrome of menopause) (6) aerobic vaginitis/desquamative inflammatory vaginitis and (7) cytolytic vaginosis
CONTACT NOW
Are you interested in the product?
We’d love to hear from you. Get in touch with us just below to request an offer!

